| Generic Drug/Consistency Evaluation Platform |
Services and Solutions
Fukangren is committed to the research and development of new drugs and the consistency evaluation of generic drugs. It has a strong team of highly educated core personnel with rich experience in new drug R&D and industrialization.
| High-end preparation platform |
| Innovative medicine/international cooperation platform |
| Clinical research and development platform |
| MAH Licensed Service Platform |


CDMO
Focus on the development of high-end preparations and international cooperation in...
| R&D Center |
| Industry Base |

| Project Management |
| Quality Assurance |

About Us
Beijing Fukangren Biopharmaceutical Technology Co., Ltd. started its drug R&D service in 1999 and is the CRO company with the longest continuous service time in China. Owns 3 pharmaceutical R&D centers, 1 clinical R&D center, 4 API production bases and 1 preparation production base
| About Us |
| Development Path |
| Company Culture |
| Company Team |
| Business Planning |


Contact Us
Sound organization, high-quality quality management team, complete document management system...
| Business Cooperation |
| Talent Recruitment |

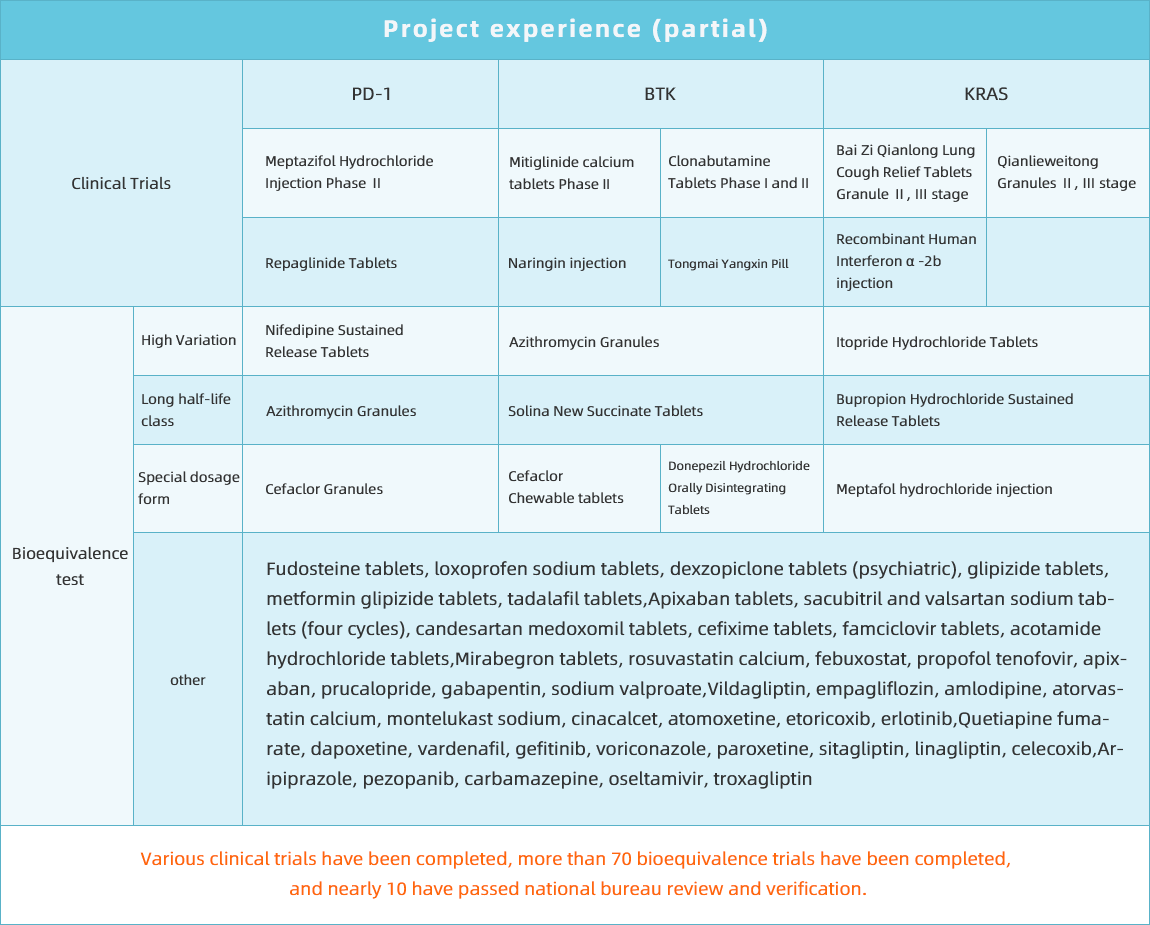
Clinical research and development platform
Committed to research and development of new drugs and consistency evaluation of generic drugs
Can undertake business
![]()
Bioequivalence test
Clinical trials for various indications
Clinical biological sample testing
Bioanalytical methodology development, transfer and verification

Quality Goal
![]()
· Customer satisfaction ≥ 90 points
· Timely completion rate of audits 100%
· The implementation rate of personnel training reached 100%
· The instrument and equipment verification and calibration rate reached 100%
· 100% of inspection tasks completed on time
ISR pass rate ≥ 90%

Test site, environment and facilities

Liquid chamber

Sample processing room

Darkroom

Balance room

Preparation room

Sample storage room
Project Experience